Prostate cancer
Adult: Metastatic, castration-resistant prostate cancer: 1 g once daily, in combination w/ prednisone or prednisolone.
|
Indications and Dosage
Oral
Prostate cancer Adult: Metastatic, castration-resistant prostate cancer: 1 g once daily, in combination w/ prednisone or prednisolone.
|
|
Hepatic Impairment
Moderate (Child-Pugh Class B): 250 mg once daily; permanently discontinue if ALT/AST reaches >5 times the upper limit of normal (ULN) or total bilirubin >3 times ULN during treatment. Severe (Child-Pugh Class C): Contraindicated.
|
|
Administration
Should be taken on an empty stomach. Take at least 1 hr before or 2 hr after meals. Swallow whole, do not chew/crush.
|
|
Contraindications
Severe hepatic impairment (Child-Pugh Class C). Pregnancy and lactation.
|
|
Special Precautions
Patient w/ ventricular arrhythmia, heart failure, recent MI, severe or unstable angina, DM, history of CV disease. Moderate hepatic and severe renal impairment.
|
|
Adverse Reactions
Significant: Adrenocortical insufficiency, mineralocorticoid excess (often leads to fluid retention, hypokalaemia, HTN, and cardiac failure), hyperglycaemia, anaemia, myopathy, rhabdomyolysis, decreased bone density, sexual dysfunction.
Nervous: Fatigue. CV: Angina pectoris, arrhythmias (e.g. AF, tachycardia), chest pain, oedema, hot flush. GI: Diarrhoea, dyspepsia. Resp: Upper resp tract infection, cough. Hepatic: Increased transaminase and bilirubin levels. Genitourinary: Nocturia, urinary frequency, UTI. Endocrine: Hypertriglyceridaemia, hypophosphataemia. Musculoskeletal: Muscular discomfort, joint swelling, fractures. Immunologic: Sepsis. Dermatologic: Rash. Potentially Fatal: Hepatotoxicity (e.g. acute liver failure, fulminant hepatitis). |
|
Monitoring Parameters
Monitor BP, serum K level, and fluid balance prior to and at least mthly during therapy; LFT prior to therapy, then 2 wkly on the 1st 3 mth, then mthly thereafter. Monitor signs and symptoms of adrenocorticoid insufficiency.
|
|
Drug Interactions
Increased risk of hypokalaemia w/ K-lowering drugs (e.g. thiazide diuretics). Risk of tumour progression w/ spironolactone. Decreased exposure w/ strong CYP3A4 inducers (e.g. rifampicin). Increased plasma concentration w/ potent CYP3A4 inhibitors e.g. ketoconazole. Increased risk of QT prolongation w/ antiarrhythmics (e.g. quinidine, amiodarone), antipsychotics, moxifloxacin, methadone. May increase exposure to drugs that are metabolised or activated by CYP2D6 (e. g. desipramine, venlafaxine, metoprolol), esp those w/ narrow therapeutic index.
|
|
Food Interaction
Increased absorption w/ food. Decreased absorption w/ St John’s wort. Increased plasma concentration w/ grapefruit or grapefruit juice.
|
|
Action
Description: Abiraterone suppresses testosterone production through selective and irreversible inhibition of 17 α-hydroxylase/C17, 20-lyase (CYP17), an enzyme required for androgen biosynthesis in testicular, adrenal, and prostatic tumour tissues.
Pharmacokinetics: Absorption: Increased absorption w/ food. Time to peak plasma concentration: Approx 2 hr. Distribution: Plasma protein binding: >99%, to albumin and α1-acid glycoprotein. Metabolism: Metabolised in the liver via hydroxylation, oxidation, and sulfation by CYP3A4 and sulfotransferase (SULT2A1) enzymes, forming 2 major inactive metabolites, abiraterone sulfate and N-oxide abiraterone sulfate. Excretion: Via faeces (88%) and urine (approx 5%). Terminal elimination half-life: Approx 15 hr. |
|
Chemical Structure
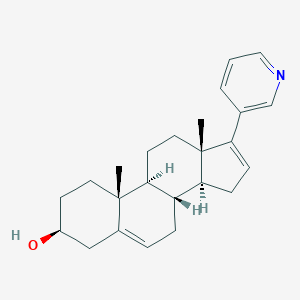 Source: National Center for Biotechnology Information. PubChem Database. Abiraterone, CID=132971, https://pubchem.ncbi.nlm.nih.gov/compound/Abiraterone (accessed on Jan. 20, 2020) |
|
Storage
Store between 20-25°C.
|
|
MIMS Class
|
|
ATC Classification
L02BX03 - abiraterone ; Belongs to the class of other hormone antagonists and related agents. Used in the treatment of metastatic castration-resistant prostate cancer.
|
|
References
Anon. Abiraterone Acetate . Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 03/05/2017. Buckingham R (ed). Abiraterone Acetate. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 03/05/2017. Joint Formulary Committee. Abiraterone Acetate. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 03/05/2017. McEvoy GK, Snow EK, Miller J et al (eds). Abiraterone Acetate. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 03/05/2017. Zytiga Tablets (Janssen Biotech, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 03/05/2017.
|