Muscle spasms, Post-stroke spasticity
Adult: 50-150 mg tid.
|
Indications and Dosage
Oral
Muscle spasms, Post-stroke spasticity Adult: 50-150 mg tid.
|
|
Renal Impairment
Severe: Not recommended.
|
|
Hepatic Impairment
Severe: Not recommended.
|
|
Administration
Should be taken with food.
|
|
Contraindications
Hypersensitivity to tolperisone or the similar drug, eperisone. Myasthenia gravis.
|
|
Special Precautions
Patient with hypersensitivity to lidocaine or other drugs, epilepsy. Females. Hepatic or renal impairment. Pregnancy and lactation.
|
|
Adverse Reactions
Gastrointestinal disorders: Abdominal pain, diarrhoea, nausea.
General disorders and administration site conditions: Weakness. Immune system disorders: Hypersensitivity reactions (e.g. erythema, rash, urticaria, pruritus). Musculoskeletal and connective tissue disorders: Muscle pain. Nervous system disorders: Headache, dizziness, tremors. Psychiatric disorders: Rarely, confusion. Skin and subcutaneous tissue disorders: Rarely, increased sweating. Potentially Fatal: Very rarely, severe systemic hypersensitivity reactions (e.g. anaphylactic shock). |
|
Patient Counseling Information
This drug may cause dizziness, somnolence, attention disturbance, epilepsy, blurred vision or muscular weakness; if affected, do not drive or operate machinery.
|
|
Drug Interactions
Concomitant use with methocarbamol may result in accommodation disorder of the eyes. Increased sedative effect with other centrally-acting muscle relaxants. May increase the blood levels of drugs that are metabolised by CYP2D6 (e.g. thioridazine, tolterodine, venlafaxine, atomoxetine, desipramine, dextromethorphan, metoprolol, nebivolol, perphenazine). Potentiates the effect of niflumic acid or other NSAIDs.
|
|
Food Interaction
Increased bioavailability with food.
|
|
Action
Description:
Mechanism of Action: Tolperisone is a centrally acting muscle relaxant that inhibits mono- and polysynaptic reflex transmission by both pre-synaptic and post-synaptic mechanisms. It stabilises neuronal membranes by decreasing the influx of Na and the amplitude and frequency of action potentials, it inhibits voltage-dependent Ca channels thus reducing transmitter release, and decreases the reticulo-spinal facilitation in the brainstem. Pharmacokinetics: Absorption: Rapidly absorbed. Bioavailability: 17%; increased with a high-fat meal. Time to peak plasma concentration: 30-60 minutes. Distribution: Volume of distribution: 5 L/kg. Metabolism: Metabolised in the liver mainly by the CYP2D6 and to a lesser extent CYP2C19, CYP2B6, and CYP1A2 isoenzymes to 11 metabolites. Excretion: Via urine (98%, as unchanged drug and metabolites). Elimination half-life: 1.5-2.5 hours. |
|
Chemical Structure
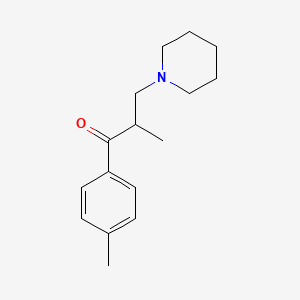 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 5511, Tolperisone. https://pubchem.ncbi.nlm.nih.gov/compound/Tolperisone. Accessed Feb. 23, 2022. |
|
Storage
Store below 25°C. Protect from heat and light.
|
|
MIMS Class
|
|
ATC Classification
M03BX04 - tolperisone ; Belongs to the class of other centrally-acting muscle relaxants.
|
|
References
Anon. Tolperisone. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 18/11/2021. Biocalm (Biolab Co., Ltd.). MIMS Thailand. http://www.mims.com/thailand. Accessed 18/11/2021. Buckingham R (ed). Tolperisone Hydrochloride. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 18/11/2021. Musolax F.C. Tablets 150 mg (Synmosa Biopharma Hong Kong Company Limited). MIMS Hong Kong. http://www.mims.com/hongkong. Accessed 18/11/2021. Product Information as Approved by the CHMP on 18 October 2012, Pending Endorsement by the European Commission. European Medicines Agency [online]. Accessed 18/11/2021. Tolperisone. European Medicines Agency [online]. Accessed 18/11/2021.
|