Amyotrophic lateral sclerosis
Adult: 50 mg bid. Discontinue if ALT levels increase to 5 times the upper limit of normal (ULN).
|
Indications and Dosage
Oral
Amyotrophic lateral sclerosis Adult: 50 mg bid. Discontinue if ALT levels increase to 5 times the upper limit of normal (ULN).
|
|
Hepatic Impairment
Contraindicated.
|
|
Administration
Should be taken on an empty stomach.
|
|
Contraindications
Hepatic impairment (baseline transaminases >3 times the upper limit of normal).
|
|
Special Precautions
Patient with history of liver diseases. Pregnancy and lactation.
|
|
Adverse Reactions
Significant: Neutropenia, dizziness, drowsiness, vertigo, interstitial lung disease.
Cardiac disorders: Tachycardia. Gastrointestinal disorders: Oral paraesthesia, diarrhoea, abdominal pain, nausea, vomiting. General disorders and administration site conditions: Asthenia, pain. Immune system disorders: Angioedema, anaphylactoid reaction. Investigations: Abnormal LFT. Nervous system disorders: Headache. Potentially Fatal: Hepatic injury. |
|
Patient Counseling Information
This drug may cause dizziness, drowsiness or vertigo, if affected, do not drive or operate machinery.
|
|
Monitoring Parameters
Monitor serum aminotransferase concentrations (e.g. ALT) before and during therapy; signs and symptoms of hepatic injury.
|
|
Overdosage
Symptoms: Methaemoglobinaemia, memory loss, acute toxic encephalopathy with stupor and coma. Management: Symptomatic and supportive treatment.
|
|
Drug Interactions
Decreased rate of elimination with CYP1A2 inhibitors (e.g. caffeine, ciprofloxacin, oral contraceptives). Increased rate of elimination with CYP1A2 inducers (e.g. rifampicin, omeprazole). Increased risk of hepatotoxicity with hepatotoxic drugs (e.g. allopurinol, methyldopa, sulfasalazine).
|
|
Food Interaction
Decreased absorption with high-fat meals. Increased elimination with charcoal-broiled food.
|
|
Action
Description:
Mechanism of Action: Riluzole is a glutamate inhibitor used to slow disease progression and prolong survival rate in patients with amyotrophic lateral sclerosis. The exact mechanism of its action is not fully elucidated but is shown to inhibit the release of glutamate, inactivate voltage-dependent Na channels, and interfere with intracellular events following binding of transmitter at excitatory amino acid receptors. Pharmacokinetics: Absorption: Rapidly absorbed from the gastrointestinal tract. High-fat meal decreases the rate and extent of absorption. Absolute bioavailability: Approx 60%. Time to peak plasma concentration: 1 to 1.5 hours. Distribution: Widely distributed throughout the body. It crosses the blood-brain barrier. Volume of distribution: Approx 3.4 L/kg. Plasma protein binding: Approx 97%, mainly to albumin and lipoproteins. Metabolism: Extensively metabolised in the liver mainly via oxidation by CYP1A2 isoenzyme to the major active metabolite, N-hydroxy-riluzole; undergoes subsequent glucuronidation. Excretion: Via urine (90%, >85% as glucuronides and 2% as unchanged drug): via faeces (5%). Elimination half-life: Approx 9-15 hours. |
|
Chemical Structure
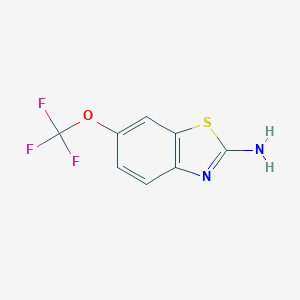 Source: National Center for Biotechnology Information. PubChem Database. Riluzole, CID=5070, https://pubchem.ncbi.nlm.nih.gov/compound/Riluzole (accessed on Jan. 23, 2020) |
|
Storage
Store between 20-25°C. Protect from light.
|
|
ATC Classification
N07XX02 - riluzole ; Belongs to the class of other nervous system drugs.
|
|
References
Anon. Riluzole. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 12/11/2018. Anon. Riluzole. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 12/11/2018. Buckingham R (ed). Riluzole. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 12/11/2018. Riluzole (Ascend Laboratories, LLC). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 12/11/2018.
|