Idiopathic parkinsonism
Adult: 1 mg once daily as monotherapy or as adjunctive therapy to levodopa.
|
Indications and Dosage
Oral
Idiopathic parkinsonism Adult: 1 mg once daily as monotherapy or as adjunctive therapy to levodopa.
|
|
Hepatic Impairment
Mild: 0.5 mg once daily.
|
|
Administration
May be taken with or without food. Avoid tyramine-rich foods, beverages or dietary supplements & amines (from cough/cold prep).
|
|
Contraindications
Severe hepatic impairment. Concomitant use w/ other MAOIs and pethidine w/in 14 days after discontinuation of therapy; St John's wort.
|
|
Special Precautions
Mild to moderate hepatic impairment. Pregnancy and lactation
|
|
Adverse Reactions
Headache, flu-like syndrome, malaise, neck pain, angina pectoris, dyspepsia, anorexia, leucopenia, arthralgia, arthritis, depression, vertigo, rhinitis, conjunctivitis, skin rashes, melanoma, and urinary urgency. Rarely, CVA and MI.
|
|
Patient Counseling Information
Avoid tobacco smoking.
|
|
Monitoring Parameters
Monitor BP, symptoms of parkinsonism, new or worsening mental status and behavioural changes, somnolence and falling asleep during daily activities and skin examination for presence of melanoma.
|
|
Overdosage
Symptoms: Dysphoria, hypomania, hypertensive crisis and serotonin syndrome. Management: Symptomatic and supportive therapy.
|
|
Drug Interactions
Increased plasma levels w/ potent CYP1A2 inhibitors (e.g. ciprofloxacin). Increased clearance w/ entacapone.
Potentially Fatal: Increased risk of non-selective MAO inhibition w/ other MAOIs and pethidine that may lead to hypertensive crises. |
|
Food Interaction
Increased risk of hypertensive crises w/ St John's wort. May reduce plasma concentration w/ tobacco smoking.
|
|
Action
Description:
Mechanism of Action: Rasagiline is a potent, irreversible monoamine oxidase (MAO)-B selective inhibitor which may cause an increase in extracellular levels of dopamine in the striatum, leading to reduced symptomatic motor deficits of Parkinson's disease. Duration: Approx 1 wk (irreversible inhibition). Pharmacokinetics: Absorption: Rapidly absorbed from the GI tract. Absolute bioavailability: Approx 36%. Time to peak plasma concentration: Approx 30-60 min. Distribution: Plasma protein binding: Approx 60-70%. Metabolism: Undergoes extensive hepatic metabolism via N-dealkylation and/or hydroxylation to yield 1-aminoindan (major metabolite), 3-hydroxy-N-propargyl-1 aminoindan and 3-hydroxy-1-aminoindan by CYP1A2 isoenzyme, and conjugation to yield glucuronides. Excretion: Mainly in the urine (<1% as unchanged drug) and partly in the faeces. Terminal half-life: 0.6-2 hr. |
|
Chemical Structure
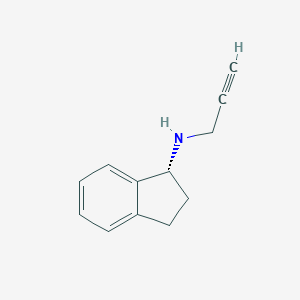 Source: National Center for Biotechnology Information. PubChem Database. Rasagiline, CID=3052776, https://pubchem.ncbi.nlm.nih.gov/compound/Rasagiline (accessed on Jan. 22, 2020) |
|
Storage
Store below 25°C.
|
|
MIMS Class
|
|
ATC Classification
N04BD02 - rasagiline ; Belongs to the class of dopaminergic agents, monoamine oxidase B inhibitors. Used in the management of Parkinson's disease.
|
|
References
Anon. Rasagiline. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 04/04/2016. Buckingham R (ed). Rasagiline Mesilate. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 01/04/2016. Joint Formulary Committee. Rasagiline. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 01/04/2016. McEvoy GK, Snow EK, Miller J et al (eds). Rasagiline Mesylate. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 01/04/2016.
|