Hypertension
Adult: 20 mg daily as a single dose or in 2 divided doses; may be increased to 20 mg bid as necessary.
Elderly: Initially, 10 mg daily.
Elderly: Initially, 10 mg daily.
|
Indications and Dosage
Oral
Hypertension Adult: 20 mg daily as a single dose or in 2 divided doses; may be increased to 20 mg bid as necessary.
Elderly: Initially, 10 mg daily. |
|
Hepatic Impairment
Initially, 10 mg daily.
|
|
Administration
Should be taken with food.
|
|
Contraindications
Pregnancy and lactation.
|
|
Special Precautions
Patient with cardiogenic shock or severe aortic stenosis. Hepatic impairment. Elderly.
|
|
Adverse Reactions
Eye disorders: Eye pain.
Gastrointestinal disorders: Nausea, gingival hyperplasia, gastrointestinal disturbance. General disorders and administration site conditions: Lethargy, peripheral oedema. Nervous system disorders: Dizziness, headache. Psychiatric disorders: Depression. Renal and urinary disorders: Increased micturition frequency. Vascular disorders: Hypotension, flushing. |
|
Drug Interactions
Increased plasma concentration with cimetidine and ranitidine. Decreased plasma concentration and exposure with bile acids (e.g. ursodeoxycholic acid, chenodeoxycholic acid).
|
|
Food Interaction
Grapefruit juice may increase the bioavailability of nitrendipine.
|
|
Action
Description:
Mechanism of Action: Nitrendipine is a dihydropyridine Ca channel blocker. It inhibits the influx of Ca ions through a voltage-dependent Ca channel in vascular smooth muscle cells. Pharmacokinetics: Absorption: Well absorbed from the gastrointestinal tract. Absolute bioavailability: Approx 10-30%. Time to peak plasma concentration: Within 1-3 hours. Distribution: Plasma protein binding: Approx 98%. Metabolism: Extensively metabolised in the liver. Undergoes extensive first-pass metabolism. Excretion: Via urine and faeces (as inactive metabolites, <0.1% as unchanged drug). Terminal elimination half-life: Approx 10-22 hours. |
|
Chemical Structure
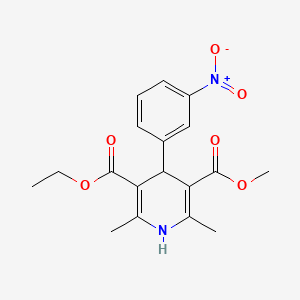 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 4507, Nitrendipine. https://pubchem.ncbi.nlm.nih.gov/compound/Nitrendipine. Accessed Feb. 27, 2023. |
|
Storage
Store below 25°C.
|
|
MIMS Class
|
|
ATC Classification
C08CA08 - nitrendipine ; Belongs to the class of dihydropyridine derivative selective calcium-channel blockers with mainly vascular effects. Used in the treatment of cardiovascular diseases.
|
|
References
Triggle DJ. Antihypertensive Mechanism of Action and Binding Sites of Nitrendipine. The American Journal of Cardiology. 1986 Sep;58(8):D35-D38. https://doi.org/10.1016/0002-9149(86)90423-6. Accessed 20/02/2023 Anon. Nitrendipine. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 15/11/2022. Buckingham R (ed). Nitrendipine. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 15/11/2022. Ditrenil (Siam Bheasach). MIMS Thailand. http://www.mims.com/thailand. Accessed 15/11/2022. Preston CL (ed). Calcium-channel Blockers + Grapefruit Juice. Stockley’s Drug Interactions [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 15/11/2022. Preston CL (ed). Calcium-channel Blockers + H2-receptor Antagonists. Stockley’s Drug Interactions [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 20/02/2023. Preston CL (ed). Calcium-channel Blockers; Nitrendipine + Bile Acids. Stockley’s Drug Interactions [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 15/11/2022.
|