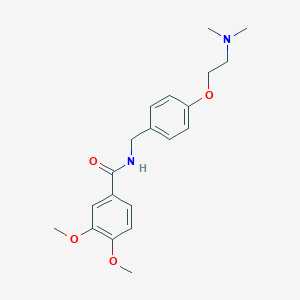
Disorders associated with reduced gastrointestinal motility
Adult: For the management of gastrointestinal symptoms including the feeling of swollen abdomen, early satiety, postprandial fullness, upper abdominal pain or discomfort, nausea and vomiting, anorexia, and heartburn; non-ulcer dyspepsia: 50 mg tid. May reduce dose depending on the patient's age and symptoms.




 Sign Out
Sign Out