Cerebrovascular and peripheral vascular disease
Adult: 10-20 mg 3 or 4 times daily.
|
Indications and Dosage
Oral
Cerebrovascular and peripheral vascular disease Adult: 10-20 mg 3 or 4 times daily.
|
|
Administration
May be taken with or without food. May be taken w/ meals, milk or antacids to minimise GI discomfort.
|
|
Contraindications
Recent arterial haemorrhage.
|
|
Special Precautions
Pregnancy and lactation. Not intended to be given immediately postpartum.
|
|
Adverse Reactions
Significant: Severe rash.
Nervous: Trembling, nervousness, weakness, dizziness. CV: Palpitation, tachycardia, chest pain, hypotension, flushing. GI: Abdominal distress, nausea, vomiting, intestinal distention. |
|
IV/Parenteral/PO: C
|
|
Action
Description:
Mechanism of Action: Isoxsuprine, a β-agonist, increases muscle blood flow by directly relaxing the vascular smooth muscle. Pharmacokinetics: Absorption: Well absorbed from the GI tract. Time to peak plasma concentration: Approx 1 hr. Distribution: Crosses the placenta. Excretion: Excreted via urine as conjugates. Plasma elimination half-life: Approx 1.5 hr. |
|
Chemical Structure
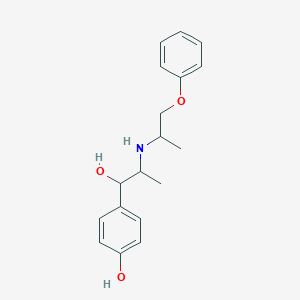 Source: National Center for Biotechnology Information. PubChem Database. Isoxsuprine, CID=3783, https://pubchem.ncbi.nlm.nih.gov/compound/Isoxsuprine (accessed on Jan. 21, 2020) |
|
Storage
Store between 15-30°C.
|
|
ATC Classification
C04AA01 - isoxsuprine ; Belongs to the class of 2-amino-1-phenylethanol derivative agents. Used as peripheral vasodilators.
|
|
References
Anon. Isoxsuprine. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 10/07/2017. Buckingham R (ed). Isoxsuprine Hydrochloride. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 10/07/2017. Isoxsuprine Hydrochloride Tablets (Vista Pharmaceuticals, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 10/07/2017. McEvoy GK, Snow EK, Miller J et al (eds). Isoxsuprine Hydrochloride. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 10/07/2017.
|