HIV-1 infection
Adult: In combination with other antiretroviral agents: 800 mg 8 hourly.
|
Indications and Dosage
Oral
HIV-1 infection Adult: In combination with other antiretroviral agents: 800 mg 8 hourly.
|
|
Special Patient Group
Patients taking delavirdine 400 mg tid: May decrease the dose to 600 mg 8 hourly.
Patients taking itraconazole 200 mg bid: May decrease the dose to 600 mg 8 hourly. Patients taking ketoconazole: May decrease the dose to 600 mg 8 hourly. Patients taking rifabutin: May increase the dose to 1,000 mg 8 hourly. Dose reduction of rifabutin to half of the standard dose is recommended (refer to specific product guidelines). |
|
Hepatic Impairment
Mild to moderate (due to cirrhosis): 600 mg 8 hourly.
|
|
Contraindications
Hypersensitivity. Lactation. Concomitant administration with drugs in which inhibition of CYP3A4 can result in elevated serum concentration of these drugs, potentially causing serious adverse reactions; St. John's wort.
|
|
Special Precautions
Patient with diabetes, haemophilia A or B. Hepatic impairment. Pregnancy. Patients taking delavirdine, itraconazole, ketoconazole or rifabutin.
|
|
Adverse Reactions
Significant: Fat redistribution (e.g. buffalo hump, peripheral wasting with increased abdominal girth, cushingoid appearance), indirect hyperbilirubinaemia, immune reconstitution syndrome; nephrolithiasis or urolithiasis (sometimes associated with renal impairment, acute renal failure or pyelonephritis), tubulointerstitial nephritis (in patients with asymptomatic severe leukocyturia); new-onset diabetes mellitus or hyperglycaemia, or exacerbation of pre-existing diabetes mellitus, diabetic ketoacidosis.
Gastrointestinal disorders: Abdominal pain, nausea, vomiting, diarrhoea, dysgeusia, dyspepsia, acid regurgitation, flatulence, dry mouth. General disorders and administration site conditions: Asthenia, fatigue. Investigations: Increased ALT and AST, decreased neutrophil counts, increased mean corpuscular volume (MCV). Musculoskeletal and connective tissue disorders: Myalgia. Nervous system disorders: Headache, dizziness, hypoaesthesia, paraesthesia. Psychiatric disorders: Insomnia. Renal and urinary disorders: Dysuria, crystalluria, haematuria, proteinuria. Skin and subcutaneous tissue disorders: Pruritus, dry skin, rash. Potentially Fatal: Acute haemolytic anaemia; hepatitis (which may result in hepatic failure). |
|
Patient Counseling Information
Ensure adequate hydration.
|
|
Monitoring Parameters
Monitor viral load, CD4 count, triglycerides, cholesterol, glucose, LFTs, CBC, urinalysis; signs of gastrointestinal disturbances that can result in dehydration and weight loss, hyperlipidaemia, redistribution of body fat, rash, CNS effects (e.g. insomnia, malaise, abnormal thinking), electrolyte imbalance.
|
|
Overdosage
Symptoms: Renal effects (e.g. flank pain, nephrolithiasis, haematuria), gastrointestinal effects (e.g. nausea, vomiting, diarrhoea). Management: Symptomatic and supportive treatment.
|
|
Drug Interactions
May increase serum concentration with CYP3A4 inhibitors, delavirdine, itraconazole, ketoconazole. May decrease serum concentration with other CYP3A4 inducers (e.g. phenobarbital, phenytoin, carbamazepine, dexamethasone), didanosine, efavirenz, nevirapine, rifabutin, venlafaxine. May increase risk of nephrolithiasis with ritonavir. May increase serum concentration of ciclosporin, tacrolimus, sirolimus, CYP3A4 inhibitors, Ca channel blockers, trazodone, parenteral midazolam, other drugs metabolised by CYP3A4. May increase risk of myopathy with other HMG-CoA reductase inhibitors (e.g. rosuvastatin, atorvastatin, pravastatin, fluvastatin). May increase risk of phosphodiesterase type 5 (PDE5) inhibitor-associated adverse effects (e.g. hypotension, visual changes, priapism) with PDE5 inhibitors (e.g. tadalafil, vardenafil, sildenafil).
Potentially Fatal: Increased risk of hypotension with alfuzosin. Increased risk of cardiac arrhythmias with amiodarone, pimozide, cisapride. Increased risk of ergot toxicity with ergot derivatives (e.g. dihydroergotamine, ergonovine, ergotamine, methylergonovine). Increased risk for myopathy (including rhabdomyolysis) with lovastatin, simvastatin. Increased risk of sedation or respiratory depression with alprazolam, midazolam (oral), triazolam. Increased risk of sildenafil-associated adverse effects with sildenafil (when used for the treatment of pulmonary arterial hypertension). May increase risk of indirect hyperbilirubinaemia with atazanavir. Increases serum concentration of astemizole, terfenadine, lurasidone. Decreases serum concentration leading to loss of virologic response with rifampicin. |
|
Food Interaction
Decreased serum concentration leading to loss of virologic response with St. John's wort; avoid concomitant use. May decrease bioavailability with food; significantly reduced serum concentration with high calorie, fat, protein meals.
|
|
Action
Description:
Mechanism of Action: Indinavir is a selective, competitive and reversible inhibitor of HIV protease. It binds to the site of HIV-1 protease activity and prevents cleavage of viral Gag-Pol polyprotein precursors, thereby resulting in the formation of immature and non-infectious viral particles. Pharmacokinetics: Absorption: Rapidly absorbed from the gastrointestinal tract. May decrease bioavailability with food; reduced absorption with high calorie, fat, protein meals. Bioavailability: Approx 65%. Time to peak plasma concentration: 0.8 ± 0.3 hours. Distribution: Crosses placenta (minimal) and enters breast milk. Plasma protein binding: Approx 60%. Metabolism: Metabolised in the liver via oxidative metabolism by CYP3A4 isoenzyme and glucuronidation into inactive metabolites where 7 metabolites (6 oxidative and 1 glucuronide conjugate metabolites) have been identified. Excretion: Mainly via faeces (83%; 19.1% as unchanged drug); urine (approx 19%; 9.4% as unchanged drug). Elimination half-life: 1.8 ± 0.4 hours. |
|
Chemical Structure
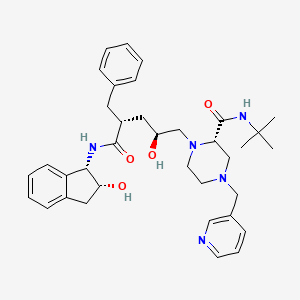 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 5362440, Indinavir. https://pubchem.ncbi.nlm.nih.gov/compound/Indinavir. Accessed Aug. 26, 2021. |
|
Storage
Store between 15-30°C. Protect from moisture.
|
|
MIMS Class
|
|
ATC Classification
J05AE02 - indinavir ; Belongs to the class of protease inhibitors. Used in the systemic treatment of viral infections.
|
|
References
Anon. Indinavir. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 03/06/2021. Anon. Indinavir. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 03/06/2021. Buckingham R (ed). Indinavir Sulfate. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 03/06/2021. Crixivan 200 mg Hard Capsules (Merck Sharp & Dome B.V.). European Medicines Agency [online]. Accessed 16/07/2021. Crixivan Capsules (Merck Sharp & Dohme Corp.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed. Accessed 03/06/2021.
|