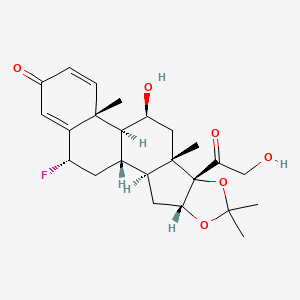
Perennial allergic rhinitis, Seasonal allergic rhinitis
Adult: As 0.25 mg (0.025%) nasal solution: Initially, 2 sprays (0.05 mg) in each nostril bid (total of 0.2 mg daily); may increase to 2 sprays in each nostril tid (total of 0.3 mg daily). Max: 8 sprays in each nostril daily (total of 0.4 mg daily).
Child: 6-14 years Initially, 1 spray (0.025 mg) in each nostril tid or 2 sprays (0.05 mg) in each nostril bid (total 0.15-0.2 mg daily). Max: 4 sprays in each nostril daily (total of 0.2 mg daily). ≥15 years Same as adult dose. Once symptoms are controlled, reduce maintenance dose to the lowest effective dose (e.g. 1 spray in each nostril once daily; total of 0.05 mg daily).
Child: 6-14 years Initially, 1 spray (0.025 mg) in each nostril tid or 2 sprays (0.05 mg) in each nostril bid (total 0.15-0.2 mg daily). Max: 4 sprays in each nostril daily (total of 0.2 mg daily). ≥15 years Same as adult dose. Once symptoms are controlled, reduce maintenance dose to the lowest effective dose (e.g. 1 spray in each nostril once daily; total of 0.05 mg daily).




 Sign Out
Sign Out