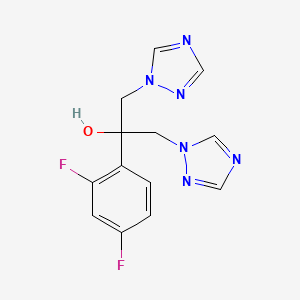
Cryptococcal meningitis
Adult: As treatment: 400 mg on Day 1, followed by 200-400 mg once daily; may increase to 800 mg daily if needed. Duration of treatment: Minimum of 6-8 weeks. For prevention of relapse after primary course of treatment for cryptococcal meningitis in patients with AIDS: 200 mg once daily. Doses are given via infusion. Infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: 6-12 mg/kg once daily, depending on the severity of infection. For prevention of relapse in patients with AIDS: 6 mg/kg once daily. Doses are given via infusion. Infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: 6-12 mg/kg once daily, depending on the severity of infection. For prevention of relapse in patients with AIDS: 6 mg/kg once daily. Doses are given via infusion. Infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Intravenous
Oesophageal candidiasis
Adult: 50 mg once daily, may increase to 100 mg once daily if needed. Duration of treatment: 14-30 days. Alternatively, an initial dose of 200 mg on Day 1, followed by 100 mg once daily; may increase up to 400 mg daily. Duration of treatment: Minimum of 3 weeks and for at least 2 weeks after symptom resolution. Doses are given via infusion. Dosage and infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: 6 mg/kg on Day 1, followed by 3 mg/kg once daily. Doses are given via infusion. Infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: 6 mg/kg on Day 1, followed by 3 mg/kg once daily. Doses are given via infusion. Infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Intravenous
Oropharyngeal candidiasis
Adult: 50-100 mg once daily for 7-14 days. Alternatively, an initial dose of 200 mg on Day 1, followed by 100 mg once daily for at least 2 weeks. Doses are given via infusion. Dosage and infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: 6 mg/kg on Day 1, followed by 3 mg/kg once daily. Doses are given via infusion. Dosage and infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: 6 mg/kg on Day 1, followed by 3 mg/kg once daily. Doses are given via infusion. Dosage and infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Intravenous
Candiduria
Adult: 50 mg once daily, may increase to 100 mg once daily if needed. Duration of treatment: 14-30 days. Doses are given via infusion. Infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Intravenous
Chronic atrophic candidiasis
Adult: In cases associated with dentures: 50 mg once daily for 14 days given via infusion. Infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Intravenous
Prophylaxis of candidiasis
Adult: In patients at high risk of systemic infections (e.g. due to occurring potentiated or prolonged neutropenic phase): 400 mg once daily. Initiate treatment several days before the anticipated onset of neutropenia and continue for 7 days after the neutrophil count has increased to >1,000 cells/mm3. Doses are given via infusion. Treatment and infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: In immunocompromised patients: 3-12 mg/kg once daily, depending on the extent and duration of neutropenia. Doses are given via infusion. Treatment and infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: In immunocompromised patients: 3-12 mg/kg once daily, depending on the extent and duration of neutropenia. Doses are given via infusion. Treatment and infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Intravenous
Systemic candidiasis
Adult: 400 mg on Day 1, followed by 200-400 mg once daily. Duration of treatment is based on clinical response. Doses are given via infusion. Infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: 6-12 mg/kg once daily, depending on the severity of the infection. Doses are given via infusion. Infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: 6-12 mg/kg once daily, depending on the severity of the infection. Doses are given via infusion. Infusion rate recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Oral
Cryptococcal meningitis
Adult: As treatment: 400 mg on Day 1, followed by 200-400 mg once daily. Duration of treatment: Minimum of 6-8 weeks. For prevention of relapse after primary course of treatment for cryptococcal meningitis in patients with AIDS: 100-200 mg once daily.
Child: 6-12 mg/kg once daily, depending on the severity of the infection. For prevention of relapse in patients with AIDS: 6 mg/kg once daily.
Child: 6-12 mg/kg once daily, depending on the severity of the infection. For prevention of relapse in patients with AIDS: 6 mg/kg once daily.
Oral
Oropharyngeal candidiasis
Adult: 50 mg once daily, may increase to 100 mg once daily if needed. Duration of treatment: 7-14 days. Alternatively, an initial dose of 200 mg on Day 1, followed by 100 mg once daily for at least 14 days.
Child: 6 mg/kg on Day 1, followed by 3 mg/kg once daily. Max: 400 mg daily.
Child: 6 mg/kg on Day 1, followed by 3 mg/kg once daily. Max: 400 mg daily.
Oral
Oesophageal candidiasis
Adult: 50 mg once daily, may increase to 100 mg once daily if needed. Duration of treatment: 14-30 days. Alternatively, an initial dose of 200 mg on Day 1, followed by 100 mg once daily; may increase up to 400 mg daily if needed. Duration of treatment: Minimum of 3 weeks and for at least 2 weeks after symptom resolution. Dosage recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: 6 mg/kg on Day 1, followed by 3 mg/kg once daily; may increase up to 12 mg/kg daily if needed. Duration of treatment: Minimum of 3 weeks and for at least 2 weeks after symptom resolution. Dosage recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: 6 mg/kg on Day 1, followed by 3 mg/kg once daily; may increase up to 12 mg/kg daily if needed. Duration of treatment: Minimum of 3 weeks and for at least 2 weeks after symptom resolution. Dosage recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Oral
Non-invasive bronchopulmonary candidiasis
Adult: 50 mg once daily, may increase to 100 mg once daily if needed. Duration of treatment: 14-30 days.
Oral
Chronic atrophic candidiasis
Adult: In cases associated with dentures: 50 mg once daily for 14 days.
Oral
Cutaneous candidiasis, Tinea corporis, Tinea cruris, Tinea pedis
Adult: 50 mg once daily or 150 mg once weekly. Duration of treatment: 2-4 weeks (up to 6 weeks in tinea pedis).
Oral
Systemic candidiasis
Adult: 400 mg on Day 1, followed by 200-400 mg once daily. Duration of treatment is based on clinical response.
Child: 6-12 mg/kg once daily, depending on the severity of the infection.
Child: 6-12 mg/kg once daily, depending on the severity of the infection.
Oral
Pityriasis versicolor
Adult: 50 mg once daily or 150 mg once weekly for 2-4 weeks. Alternatively, 300 mg once weekly for 2 weeks.
Oral
Candidal balanitis
Adult: 150 mg as a single dose.
Child: ≥16 years Same as adult dose.
Child: ≥16 years Same as adult dose.
Oral
Candiduria
Adult: 50 mg once daily, may increase to 100 mg once daily if needed. Duration of treatment: 14-30 days.
Oral
Vaginal candidiasis
Adult: 150 mg as a single dose. For recurrent cases: 150 mg 72 hourly for 3 doses, then 150 mg once weekly for 6 months.
Oral
Prophylaxis of candidiasis
Adult: In patients at high risk of systemic infections (e.g. due to occurring potentiated or prolonged neutropenic phase): 400 mg once daily. Initiate treatment several days before the anticipated onset of neutropenia and continue for 7 days after the neutrophil count has increased to >1,000 cells/mm3. Dosage or treatment recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: In immunocompromised patients: 3-12 mg/kg once daily, depending on the extent and duration of neutropenia. Treatment recommendations may vary among countries and individual products (refer to specific country or product guidelines).
Child: In immunocompromised patients: 3-12 mg/kg once daily, depending on the extent and duration of neutropenia. Treatment recommendations may vary among countries and individual products (refer to specific country or product guidelines).




 Sign Out
Sign Out