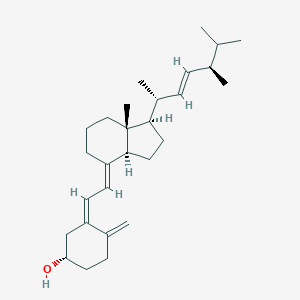
Familial hypophosphataemia, Vitamin D-resistant rickets
Adult: Dosage is individualised according to patient response and requirement. 12,000-500,000 IU daily. Alternatively, 10,000-60,000 IU daily may be given with phosphate supplements. Readjust dose as soon as clinical improvement is observed. Frequent serum Ca determination is recommended when high therapeutic doses are used.
Oral
Hypoparathyroidism
Adult: Dosage is individualised according to patient response and requirement. In combination with Ca supplements and/or IV parathyroid hormone or dihydrotachysterol: 50,000-200,000 IU daily. Readjust dose as soon as clinical improvement is observed.
Child: Same as adult dose.
Child: Same as adult dose.
Oral
Vitamin D deficiency
Adult: Dosage is individualised according to patient response and requirement. Prophylaxis: 400 IU daily. Treatment: 800 IU daily, may require higher doses in severe deficiency. Alternatively, 50,000 IU once weekly may be necessary. In patients with deficiency due to liver disease or malabsorption states: Up to 40,000 IU daily.




 Sign Out
Sign Out