Onychomycosis
Adult: For the treatment of cases caused by Trichophyton rubrum and Trichophyton mentagrophytes: As 10% solution: Apply once daily for 48 weeks.
|
Indications and Dosage
Topical/Cutaneous
Onychomycosis Adult: For the treatment of cases caused by Trichophyton rubrum and Trichophyton mentagrophytes: As 10% solution: Apply once daily for 48 weeks.
|
|
Contraindications
Hypersensitivity.
|
|
Special Precautions
Not for ophthalmic, oral, or vaginal administration. Pregnancy and lactation.
|
|
Adverse Reactions
Significant: Local pain, irritation, dermatitis.
General disorders and administration site conditions: Application site vesicles. Skin and subcutaneous tissue disorders: Ingrown toenail. |
|
Topical: C
|
|
Patient Counseling Information
Avoid pedicures, use of nail polish, and application of other nail products during treatment.
|
|
Monitoring Parameters
Monitor application site for dermatitis, vesicles, pain, and ingrown toenails.
|
|
Action
Description:
Mechanism of Action: Efinaconazole a triazole derivative, is a synthetic azole antifungal agent. It inhibits 14α-demethylase which causes a decrease in ergosterol concentrations in fungal cell membranes leading to diminished cell membrane integrity and eventually, fungal cell death. Pharmacokinetics: Absorption: Absorbed systemically after topical application (low concentrations). Excretion: Elimination half-life: 29.9 hours. |
|
Chemical Structure
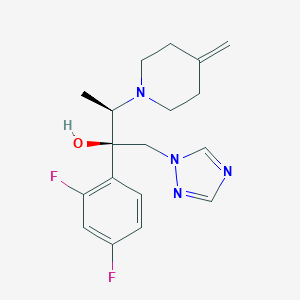 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 489181, Efinaconazole. https://pubchem.ncbi.nlm.nih.gov/compound/Efinaconazole. Accessed Apr. 26, 2021. |
|
Storage
Store between 20-25°C. Protect from heat or flame. Do not freeze.
|
|
MIMS Class
|
|
ATC Classification
D01AC19 - efinaconazole ; Belongs to the class of imidazole and triazole derivatives. Used in the topical treatment of fungal infection.
|
|
References
Anon. Efinaconazole. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 26/02/2021. Anon. Efinaconazole. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 26/02/2021. Buckingham R (ed). Efinaconazole. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 26/02/2021. Jublia Solution (Bausch Health Companies Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed. Accessed 26/02/2021. Jublia Topical Solution, 10% w/w (Kaken Pharmaceutical Co., Ltd.). MIMS Hong Kong. http://www.mims.com/hongkong. Accessed 26/02/2021.
|