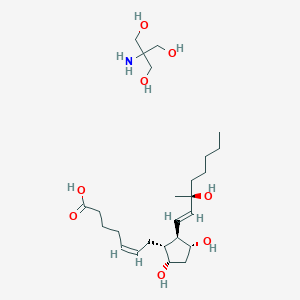
Pregnancy termination in the 2nd trimester
Adult: Initially, 250 mcg. Alternatively, initiate w/ test dose of 100 mcg. May be repeated at 1.5- to 3.5-hr intervals depending on uterine response. May be increased to 500 mcg if uterine contractility is inadequate. Max: 12 mg. Max duration: 2 days.
Intramuscular
Postpartum haemorrhage
Adult: Initially, 250 mcg by deep inj. May be repeated every 15-90-min. Max: 2 mg.




 Sign Out
Sign Out