Corticosteroid-responsive dermatoses
Adult: As 0.1% cream/oint/lotion: Apply sparingly in a thin film to affected area bid or tid, depending on the severity of the condition.
|
Indications and Dosage
Topical/Cutaneous
Corticosteroid-responsive dermatoses Adult: As 0.1% cream/oint/lotion: Apply sparingly in a thin film to affected area bid or tid, depending on the severity of the condition.
|
|
Special Precautions
Childn. Pregnancy and lactation.
|
|
Adverse Reactions
Burning, itching, soreness, stinging, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin maceration and atrophy, striae, miliaria; HPA axis and growth suppression, Cushing’s syndrome, hyperglycaemia, glucosuria.
|
|
Topical: C
|
|
Patient Counseling Information
Do not use on the face, groin, or axilla. Avoid use of occlusive dressings in the presence of infected or weeping lesions.
|
|
Monitoring Parameters
Perform urinary free cortisol and ACTH stimulation tests to monitor for hypothalamic-pituitary-adrenal (HPA) axis suppression if drug is applied to a large surface area or under an occlusive dressing.
|
|
Lab Interference
May diminish the therapeutic effect of aldesleukin, corticorelin and hyaluronidase. May enhance the hyperglycaemic effect of ceritinib. May increase the adverse/toxic effect of deferasirox.
|
|
Action
Description:
Mechanism of Action: Amcinonide, a synthetic fluorinated glucocorticoid, has anti-inflammatory, antipruritic, and vasoconstrictive actions. It induces phospholipase A2 inhibitory proteins (lipocortins) and sequentially inhibits the release of arachidonic acid, thereby depressing the formation, release, and activity of endogenous chemical mediators of inflammation. Pharmacokinetics: Absorption: Adequately absorbed through intact skin and increases w/ inflammation/occlusion. Metabolism: Metabolised in the liver. Excretion: Via urine and faeces. |
|
Chemical Structure
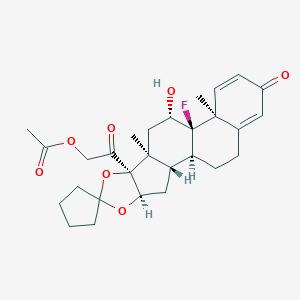 Source: National Center for Biotechnology Information. PubChem Database. Amcinonide, CID=443958, https://pubchem.ncbi.nlm.nih.gov/compound/Amcinonide (accessed on Jan. 20, 2020) |
|
Storage
Store between 15-30°C. Do not freeze.
|
|
MIMS Class
|
|
ATC Classification
D07AC11 - amcinonide ; Belongs to the class of potent (group III) corticosteroids. Used in the treatment of dermatological diseases.
|
|
References
Amcinonide Cream and Ointment (Taro Pharmaceuticals U.S.A., Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 05/09/2016. Amcinonide Lotion (Fougera Pharmaceuticals Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 05/09/2016. Anon. Amcinonide (Topical). Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 05/09/2016. Buckingham R (ed). Amcinonide. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 05/09/2016. McEvoy GK, Snow EK, Miller J et al (eds). Amcinonide (Topical). AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 05/09/2016.
|