Type 2 diabetes mellitus
Adult: As monotherapy or in combination with other antidiabetic agents: 25 mg once daily.
|
Indications and Dosage
Oral
Type 2 diabetes mellitus Adult: As monotherapy or in combination with other antidiabetic agents: 25 mg once daily.
|
||||||
|
Renal Impairment
|
||||||
|
Administration
May be taken with or without food.
|
||||||
|
Contraindications
Type 1 diabetes mellitus or diabetic ketoacidosis.
|
||||||
|
Special Precautions
Patient with abnormal LFT, history of pancreatitis, moderate to severe heart failure. Hepatic and moderate to severe renal impairment. Pregnancy and lactation. Not intended for the treatment of type 1 diabetes mellitus or diabetic ketoacidosis.
|
||||||
|
Adverse Reactions
Significant: Severe arthralgia, bullous pemphigoid, acute pancreatitis, macrovascular outcomes, heart failure.
Gastrointestinal disorders: GERD, abdominal pain, constipation. Nervous system disorders: Headache. Renal and urinary disorders: Decreased estimated GFR, renal function abnormality. Renal disease, renal impairment. Respiratory, thoracic and mediastinal disorders: Upper respiratory infection, nasopharyngitis. Skin and subcutaneous tissue disorders: Pruritus, rash. Potentially Fatal: Hepatic failure. Rarely, hypersensitivity reactions (e.g. anaphylaxis, angioedema, Steven-Johnson syndrome, erythema multiforme). |
||||||
|
Monitoring Parameters
Monitor serum glucose, glycosylated Hb (HbA1c); LFT at baseline; renal function prior to initiation of therapy and periodically thereafter; signs and symptoms of pancreatitis, hepatic and heart failure, hypersensitivity.
|
||||||
|
Drug Interactions
Increased risk of hypoglycaemia with sulfonylurea (e.g. metformin), thiazolidinedione (e.g. pioglitazone) and insulin.
|
||||||
|
Action
Description:
Mechanism of Action: Alogliptin inhibits dipeptidylpeptidase-4 (DPP-4), an enzyme that inactivates incretin hormones. Inhibition of DPP-4 result in an increase in the levels of hormones [e.g. GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotrophic polypeptide)] which regulate glucose homeostasis by increasing insulin synthesis and release from pancreatic β-cells and decreasing glucagon secretion from the pancreatic α-cells, leading to reduced hepatic glucose production. Pharmacokinetics: Absorption: Absorbed from the gastrointestinal tract. Bioavailability: Approx 100%. Time to peak plasma concentration: Approx 1-2 hours. Distribution: Volume of distribution: 417 L. Plasma protein binding: 20%. Metabolism: Minimally metabolised in the liver by CYP2D6 and CYP34A to active and inactive metabolites. Excretion: Mainly via urine (76%, 60-71% as unchanged drug); faeces (13%). Terminal elimination half-life: Approx 21 hours. |
||||||
|
Chemical Structure
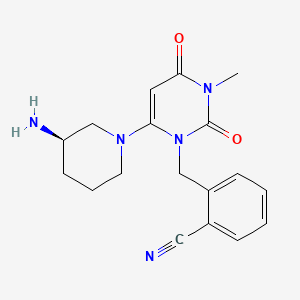 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 11450633, Alogliptin. https://pubchem.ncbi.nlm.nih.gov/compound/Alogliptin. Accessed Mar. 28, 2023. |
||||||
|
Storage
Store at 25°C.
|
||||||
|
MIMS Class
|
||||||
|
ATC Classification
A10BH04 - alogliptin ; Belongs to the class of dipeptidyl peptidase 4 (DPP-4) inhibitors. Used in the treatment of diabetes.
|
||||||
|
References
Anon. Alogliptin. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 02/02/2018. Buckingham R (ed). Alogliptin Benzoate. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 02/02/2018. Joint Formulary Committee. Alogliptin. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 02/02/2018. McEvoy GK, Snow EK, Miller J et al (eds). Alogliptin Benzoate. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 02/02/2018. Nesina Tab, Film-Coated (Takeda Pharmaceuticals America, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 02/02/2018.
|